The mechanical forces of metastasis
Professor Alpha Yap’s research team has identified a key player in the process that causes epithelial tumours to extrude cancerous cells that can go on to proliferate or invade. Their discovery offers a potential target for new drug therapies to prevent metastasis.
During the metastatic phase of epithelial cancers, mutated cells detach from a primary tumour and migrate in the surrounding tissue until they travel through the walls of blood vessels or the lymphatic system to roam the body. Sooner or later, a liberated rogue cell that evades the body’s immunosurveillance systems anchors to the wall of a blood vessel, emerges and proliferates into an aggressive secondary tumour.
Professor Alpha Yap’s team in the Molecular Cell Biology Division of the University of Queensland’s Institute for Molecular Bioscience in Brisbane have specialised in studying cadherins - the transmembrane proteins that bind cells together in organised communities.
Recently, their work took them in an unexpected direction, where they found themselves in possession of a handle on the ejector mechanism that appears to be involved in liberating rogue cells from primary cancers of epithelial tissues - a necessary prelude to metastasis.
Oncogenic extrusion
Often, cancer patients survive surgery, chemotherapy or radiotherapy to remove or ablate a primary tumour, only to succumb to aggressive, drug-resistant secondary tumours in other organs like the brain, liver or bones.
Understanding the cell-ejection mechanism, known as oncogenic extrusion, could lead to drugs that would corral cancerous cells within primary tumours, halting the metastatic process at source.
“Oncogenic extrusion is an interesting process that occurs when minorities of cancer cells are surrounded by normal or less abnormal cells,” Yap said. “Then, the surrounding cells actively expel the cancer cells from the tissue.
“This may have developed as a mechanism to clear tissues of damaged cells. But there is increasing evidence that extrusion contributes to important stages in cancer development - both early proliferation and in the earliest steps of invasion when they exit their tumour of origin.
“We didn’t start by being interested in oncogenic extrusion for its own sake; instead, this all began several years ago when we became interested in how cells regulate the mechanical processes that underpin their form and functions.”
Differing tensions within cell-cell junctions
Yap says the field of cell-to-cell adhesion is beginning to be revolutionised.
“Adhesion prevents cells becoming detached from their host tissues in response to external forces,” he explained. “But over the past five years, we’ve realised that many forces that cells experience are actually generated within the cells themselves - they are constantly pulling on the cells around them.”
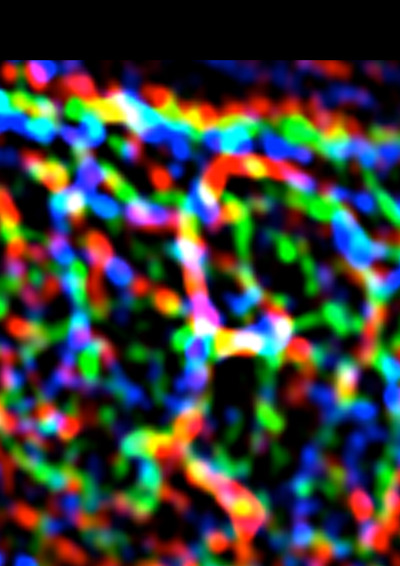
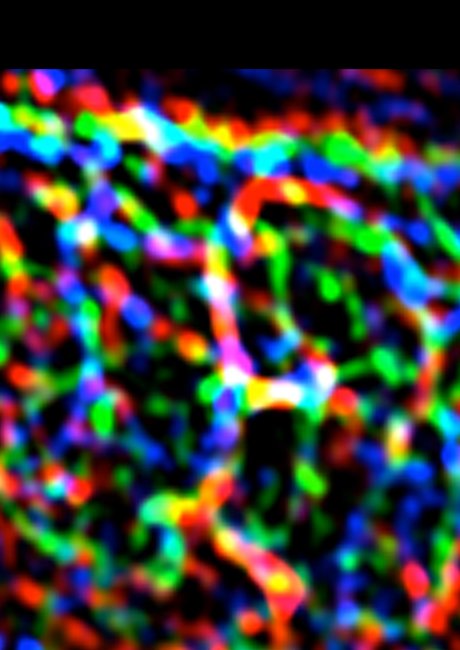
This image shows the complex patterns of E-cadherin (blue), F-actin (green) and myosin II (red) at cell-cell junctions. Image by Dr Selwin Wu.
“We found, though, that there is a pattern of pulling forces even within the junctions between cells. In many epithelial cells, E-cadherin concentrates in a chicken mesh-like pattern at the apical poles of the cells (called the zonula adherens). This is a prominent feature, but there is also a lot of E-cadherin in the junctions below the zonula adherens (which we call the lateral junctions).
“Despite this, we found that the contractile tensions generated by cells at their apical junctions were much greater than those in the lateral junctions below the zonula adherens, so even in the same cell-cell junction there are zones of differing contractile tension.”
The molecular mechanism underlying the differing tensions between apical and lateral junctions was a puzzle.
“Contractility was due to the acto-myosin cytoskeletal system, and we wondered if it was something to do with the actin,” Yap said.
Indeed, his team found that actin at the zonula adherens was more stable than actin at the lateral junctions, while stabilisation of actin filaments increased tension at the lateral junctions. This raised the issue of how actin was stabilised in the apical, but not the lateral, junctions.
A molecular handle
Coincidentally, another project in the lab had focused on a protein called N-WASP, which is related to the protein that is mutated in Wiskott-Aldrich syndrome, a rare inherited disorder involving immunodeficiency and impaired blood clotting.
N-WASP is found in almost all cells of the body and regulates the assembly of actin. Earlier, though, the Yap lab had also discovered that N-WASP stabilises actin filaments, exactly the kind of action that they were now looking for to explain tension at the zonula adherens.
“Indeed, we found that N-WASP localises very much to the zonula adherens and if you knock it down, then apical tension is reduced. Selwin Wu in my group developed a very clever strategy to target N-WASP to all parts of the junctions and found that this increased tension in the lateral junctions. This was the molecular handle that we needed,” explained Yap.
“We set out to explore the purpose of the mechanism - why would cells bother to put an actin stabiliser in one region to support higher tension in the filaments?
“Selwin then did another clever experiment, trying to change the pattern of tension in cells. He wanted to convert cells from having high apical/low lateral tension to a situation where apical tension was lower and lateral tension increased. He did this by targeting N-WASP to the lateral junctions and depleting it from the apical junctions,” Yap continued.
“When you do these kind of experiments you often have small numbers of manipulated cells that are surrounded by normal cells. We were astonished to discover that when this happened the manipulated cells became extruded from the epithelium!”
Getting the location right
Yap’s team then developed a more realistic, pathological model of oncogenic extrusion, in which they mosaically expressed the oncogene H-RAS, surrounded by normal cells. They found that N-WASP became redistributed from the apical to the lateral junctions when transformed cells contacted normal cells - exactly where extrusion occurred.
“If you then knock down N-WASP in the cell expressing the oncogene, it blocks oncogenic extrusion,” Yap said.
Yap and his colleagues published their findings in a paper in Nature Cell Biology in January this year, titled ‘Cortical F-actin stabilisation generates apical lateral patterns of junctional contractility that integrate cells into epithelia’.
“The way we believe it works is that N-WASP is normally regulated to locate within a specific region of the contact junction, generating forces that keep the cells in place among their neighbours.
“Conversely, if N-WASP is aberrantly distributed, it increases tension in places distant from the apical region, which promotes extrusion. Like real estate, if N-WASP is normally positioned, it’s good. If it’s located incorrectly, it’s bad.
Yap said they speculated that N-WASP mislocalisation may be involved in tumour progression, which may help oncogenic extrusion expel cells to allow them to proliferate or invade. Inhibiting this process might provide the basis for a useful therapy to prevent metastasis.
“Interestingly, we already have a drug that inhibits N-WASP, one that was designed in Mike Rosen’s lab as an exercise in rational drug design. So far, it has been used only in tissue culture, for research purposes, so it’s not known whether it could become a useful, deliverable therapeutic drug to inhibit dysregulation of N-WASP.”
Getting back to cell mechanics
Yap says it is interesting that oncogenic extrusion is only one of a number of examples of cell extrusion. Most occur when minorities of cells are surrounded and then expelled by relatively normal cells.
When it was originally observed in the fruit fly, Drosophila, it was described as ‘delamination’. In mammalian systems, it was identified as a mechanism associated with apoptosis - a means of clearing dead or dying cells from healthy tissues.
A couple of years ago, it was identified as a mechanism for reducing cell overcrowding in growing tissues.
“It’s not clear whether the same processes operate in all of these different forms of extrusion, because we haven’t gone very deep to identify the specific mechanisms that apply in each case,” reflected Yap.
In most cases, though, the cells to be extruded must somehow be recognised by their neighbours, which then respond.
The question, says Yap, is: what is it that the responding cells are recognising? And, in the particular case of their work, what do the neighbouring cells experience when N-WASP becomes relocalised in a transformed cell?
“Possibly it involves stiffening of the cell cortex, or mechanosensory receptors in neighbouring cells detect something happening,” he proposed.
“It’s an interesting and important question, because it highlights the importance of understanding how mechanical forces influence cell behaviour, including cell behaviour within tissues.
“Researchers originally became interested in the mechanics of cell movement and shape way back in the 1920s and 1930s, but their work largely fell out of favour with the advent of the molecular biology revolution, because we could learn so much, and much faster.
“Now, we’re going back to cell mechanics and realising that they’re more than just epiphenomena of biochemical processes within the cell,” Yap said.
“Neuroscientists have known about this for many years, through the phenomenon of mechanosensitive ion channels. But many other types of proteins, including structural elements of the cytoskeleton and motor proteins, are mechanosensitive.
“Potentially, mechanical events can be sensed by surrounding cells and tissues, via diverse mechanosensors, and trigger a response.”
Asked if the Wnt signalling system, which establishes cell polarity, might be involved in some way with regulating asymmetries in the internal tensions of cells, Yap said it was not an area in which his team worked.
But he said Emmanuel Farge’s lab at the Curie Institute had published a paper in Nature Communications last year, reporting that if Zebrafish or Drosophila embryos were physically stimulated, it activated localised morphogenetic signalling that impinged on the Wnt signalling pathway.
“Thus, mechanical elements impinge on many levels of biology - from molecules, through tissues, to organisms,” Yap concluded.

Personality influences the expression of our genes
An international research team has used artificial intelligence to show that our personalities...
Pig hearts kept alive outside the body for 24 hours
A major hurdle for human heart transplantation is the limited storage time of the donor heart...
Breakthrough antibiotic for mycobacterial infections
The antibiotic candidate, named COE-PNH2, has been optimised to target Mycobacterium...







