Managing the cancer microenvironment
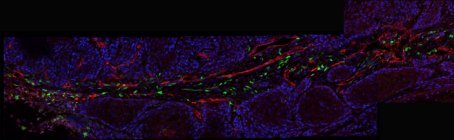
New Queensland recruit Dr Roberta Mazzieri is passionate about understanding the complex and dangerous cellular interactions that make up a tumour microenvironment. Specifically, she wants to hijack one cell component of this environment and use its cancer-promoting powers for good, not evil.
Malignant cells need to interact with and often actively recruit all other cells and structures in their surroundings to grow and prosper, and eventually to metastasise into the most deadly form of cancer. Together, these relationships make up the tumour microenvironment (TM). It is now being realised that the TM is critical for the cancer’s survival and also, in the context of therapy, a tumour’s response to treatment.
Dr Roberta Mazzieri of the Diamantina Institute in Brisbane has studied TMs for the past eight years; in particular, a subpopulation of bone marrow-derived monocytes that she helped to identify as a postdoc in Italy (reported in Blood in 2007). These white blood cells are characterised by the expression of a specific cell-surface receptor, the Tie-2 angiopoiein receptor, and thus are called Tie-2-expressing monocytes or TEMs.
Early findings reveal a potential cancer target
TEM cells, which are present in the blood of mice and humans only at very low frequency, are recruited specifically to the site of a tumour. There they differentiate into macrophages, set up shop in a highly specific pattern along the walls of surrounding blood vessels and markedly upregulate their production of the Tie-2 receptor.
It was these initial findings that first suggested to Mazzieri and her co-workers that TEMs, and their Tie-2-activated signalling pathway, could be functionally important for maintaining the TM and thus also be potential tumour markers and targets for therapy. Mazzieri has worked mostly on breast cancer, but these cells have now been isolated with several tumour types.
According to Mazzieri, one good thing about these TEMs is their characteristic Tie-2 expression because it makes them relatively easy to isolate and study.
Gene profiling of isolated TEMs and comparison to similar cell types within the TM defined them as M2 macrophages, which are typically pro-tumourigenic cells that function in tissue remodelling.
“This indicated to us that tumours might use the Tie-2 activity and remodelling abilities of TEMs to modulate their environment; for example, by promoting new blood vessel growth (angiogenesis).”
Generally, tumours need to remodel the tissue in which they are growing to make space and to induce new vessel formation to supply their often-significant energy needs.
Mazzieri’s next step was to ask what these cells actually do in the TM.
Using an existing lentiviral expression system and a transducible suicide gene linked to the Tie-2 gene promoter, they engineered a microenvironment with or without TEMs in a mouse tumour model.
“This type of specific depletion experiment showed that TEMs are absolutely required for tumour growth in vivo, because eliminating them stopped tumour growth and also strongly inhibited angiogenesis,” Mazzieri explained.
“This information, together with the gene profiling, indicated that the TEMs promote tumour angiogenesis. Moreover, TEMs isolated from humans and co-injected back into a mouse could accelerate tumour growth, suggesting a potentially critical role of TEMs in human cancer progression.”
Delving deeper into the tumour jungle
To further investigate the Tie-2 connection, Mazzieri took advantage of an established collaboration between her group in Italy and global biopharmaceutical company AstraZeneca, who have developed an anti-angiopoietin-2 antibody as an agent to inhibit tumour angiogenesis.
Angiopoietin-2 (Ang-2) is one of the main ligands for activating the Tie-2 receptor pathway and is also involved in tissue remodelling events; thus, an antibody for Ang-2 could be used to examine angiogenic pathways in the TM.
“When we treated tumour-bearing mice with the antibody, we saw a really strong inhibitory effect on tumour growth, both primary and metastatic,” explained Mazzieri, “and we saw vessel regression in the treated tumours; that is, we inhibited angiogenesis.”
Further experiments confirmed that the antibody therapy was indeed targeting the TEM cells. It was specifically disturbing their pro-angiogenic effects in the TM as well as their characteristic geographical association along the vessels. The researchers now knew for sure that this Ang2-Tie-2 signalling axis could be a powerful tumour target.
“Interestingly, around the same time, one of our collaborators showed that Ang-2 could also modulate the immunosuppressive activity of the TEMs, which they do in addition to being pro-angiogenic,” recalled Mazzieri.
Immune suppression is an important escape mechanism for tumours, helping them evade destruction by the body’s immune system and tumours do all they can to create an immunosuppressive environment. And this is where the fantastic nexus between cancer and immunology going on at the Diamantina Institute (DI) comes to the fore for Mazzieri’s work.
“Here at the DI, I plan to establish whether this anti-Ang-2 treatment, now under clinical testing, inhibits both these activities of TEMs in vivo. Such a finding would have important clinical implications because if we can somehow modulate or revert the immunosuppressive environment around a tumour by targeting the TEM activities, then we can think of combining this anti-angiogenic treatment with tumour immunotherapy that would not work otherwise due to the immunosuppression.”
Using their powers for good
A cunning second half of Mazzieri’s plan is to exploit the TEMs as cellular vehicles to deliver cancer-treating drugs directly into the sites of tumours.
“To do this we again took advantage of the cells’ own characteristics; that is, whatever TEMs produce in terms of an anti-tumour molecule will be at low levels systemically but high at the tumour site, and that is ideal. And because they are haematopoietic cells, you can isolate the stem cells from bone marrow, modify them ex vivo to express your molecule of choice and then transplant them back into a mouse or human where they will reconstitute the haematopoietic compartment and differentiate into mature TEMs.” Voila - tumour homing pigeons!
Mazzieri has already published this gene and cell-based therapy approach as a proof of principle paper using interferon-alpha as the anti-tumourigenic drug.
“By making TEMs produce interferon-alpha under the control of the Tie-2 promoter, we could inhibit the growth of primary and metastatic breast cancers in mice,” she said.
However, one of the main roadblocks to immunotherapy strategies for cancer is the availability of good preclinical models that take all the different players of the typically complex, heterogeneous and evolving tumour microenvironment into account.
Taking it into humans
To address this, Mazzieri and her team developed a humanised delivery platform for breast cancer.
Basically, they start with an immunodeficient mouse into which they transplant human haematopoietic stem cells to make a human haematopoietic compartment that produces human TEMs.
The human haematopoietic stem cells can be purified and modified ex vivo for re-injection into the mice.
“If we also inject human tumour cells into these mice we can test whether human TEMs are able to inhibit the growth of human tumours,” explained Mazzieri.
And it works a treat, according to Mazzieri, and the paper describing this research has just been accepted.
In short, they could modify the TM by making TEMs that produce interferon-alpha, which is also a pro-inflammatory protein and can thus revert the immunosuppressive microenvironment.
“Interferon-alpha activates both the innate and adaptive immune responses and we demonstrated in the mouse and in the humanised model that this seems to be enough to inhibit tumour growth,” Mazzieri said. “So, again I would like to combine this strategy with immunotherapy for future clinical use, taking advantage of the excellent tumour immunology going on here in the institute to develop a better anti-tumour metastasis therapy.
“Overall, my work involves two different approaches. In one we are targeting the activities of TEMs and in the other we are using them as a vehicle to deliver a drug,” she continued, “and in both cases, we are modulating the immunosuppressive environment surrounding a tumour, and that is why in both cases I want to combine it with immunotherapies that are either in the clinic or under development.”
Down the track
Mazzieri’s hopes going forward are that the recent push continues to reveal more about the complexity and heterogeneity of these TM interactions, for each tumour type and, more importantly, for each step in tumour progression.
“Understanding how these interactions evolve will give us the opportunity to develop more targeted and efficient therapies, particularly in the metastatic and most lethal forms of cancer.”
**********************************************************
Lorne conference line-up
Here’s the line-up for the Lorne conferences for 2014, to be held at Mantra Lorne on the Victorian south coast.
19th Lorne Proteomics Symposium
February 6-9
http://www.australasianproteomics.org/lorne-proteomics-symposium-2014/
39th Lorne Conference on Protein Structure and Function
February 9-13
http://www.lorneproteins.org/
26th Lorne Cancer Conference
February 13-15
http://www.lornecancer.org/
35th Lorne Genome Conference
February 16-19
http://www.lornegenome.org/
Lorne Infection and Immunity
February19-21
www.lorneinfectionimmunity.org
**********************************************************
Melatonin helps to prevent obesity, studies suggest
In an experiment carried out in rats, chronic administration of melatonin prevented obesity to a...
Personality influences the expression of our genes
An international research team has used artificial intelligence to show that our personalities...
Pig hearts kept alive outside the body for 24 hours
A major hurdle for human heart transplantation is the limited storage time of the donor heart...







