A handheld concussion detection device
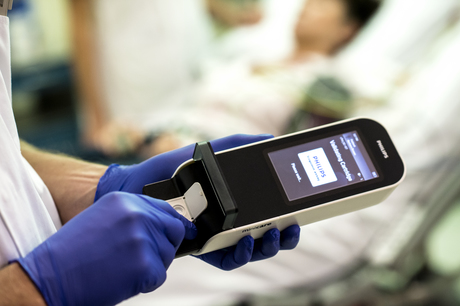
Royal Philips and Banyan Biomarkers have entered into an agreement to develop and commercialise a handheld blood test that can be used to detect mild traumatic brain injury (concussion).
According to Jordan Betel, an analyst from research and consulting firm GlobalData, the device will help to combat the considerable costs surrounding concussion — around $76.5 billion a year in the US alone.
“Costs are so high because brain trauma is currently diagnosed using radiological tests such as computed tomography [CT] and magnetic resonance imaging, which lack accuracy and timeliness,” explained Betel, with CT scans of patients’ brains often found to be normal. Failure to make a correct diagnosis and timely selection of the appropriate treatment can have serious implications, with a cascade of biological events continuing over the hours and days following a brain injury.
Currently, there is no blood test for use by physicians in the hospital to detect the presence and severity of brain trauma. Now, Banyan Biomarkers has identified two proteins that rapidly appear in the blood of patients soon after injury. A blood test to detect these proteins will be based on Philips’ Minicare I-20 system.
The product consists of a handheld analyser, dedicated software and a single-use, disposable cartridge containing the application specific test. Based on Philips’ Magnotech biosensor technology, “which concentrates, separates and detects target molecules by using magnetic nanoparticles”, according to Betel, the Minicare I-20 system is being developed to detect multiple target molecules at low concentrations within a blood sample and to display the results within minutes.
“Point-of-care diagnostics for acute care settings, such as handheld blood tests for emergency departments, will play a critical role in improving patient outcomes and reducing healthcare costs,” said Marcel van Kasteel, CEO of handheld diagnostics at Philips.
The new device is slated to hit the market in 2022, by which point Betel anticipates the in vitro diagnostic test market will be worth over $10 billion.
Pig hearts kept alive outside the body for 24 hours
A major hurdle for human heart transplantation is the limited storage time of the donor heart...
Breakthrough antibiotic for mycobacterial infections
The antibiotic candidate, named COE-PNH2, has been optimised to target Mycobacterium...
Maternal obesity may promote liver cancer in offspring
The offspring of obese female mice were found to have an 80% risk of developing cancer, compared...







